





Your Hearing Aid Manufacturer Expert
AZ Hearing is a professional manufacturer and supplier of hearing aids, hearing aid related accessories and equipment. With 5 more years of manufacturing experience, we always provide the best OEM/ODM service to our clients.
We have traditional digital, analog, BTE, ITE, CIC, and open-fit hearing aids. We are investing in applying new technologies such as Bluetooth and charging to hearing aids. AZ Hearing’s hearing aids use world-famous chip brands to ensure the best sound performance. All our hearing devices are produced under the guidance of the ISO system.
AZ Hearing can custom all types of hearing amplifiers based on your detailed requirements. Whether you need hearing aids for wholesale or distribution, we can always meet your needs.

Your Best Hearing Aid Manufacturer and Supplier
Why AZ Hearing Can Be a Reliable Hearing Aid Supplier?

5+ Years Manufacturer Experience
Over the years, we have continuously worked to improve our production efficiency and improve the quality of our products. We guarantee a high level of quality and ensure a prompt delivery time.

12 Months Warranty
AZ Hearing offer a standard 12-month factory warranty to partners. We are confident with the components used in our products.

Responsible After-Sales
We have solved distributors’ headaches with returns/repair/refurbishment for years. You could send defective hearing devices in bulk and we will take care of them.

100% Quality Inspection
Since product quality is essential to our business, all products undergo 3 rounds of differing quality checks. We only deliver products that have passed our stringent internal inspection.

Top Imported Components
Most of our hearing aids use chips from industrial leading brands which will ensure the best performance and high quality products.
Professional OEM/ODM Support
We have been offering our partners with professional labeling services since 2015. From the engraving on hearing aids body to customized packaging, we are ready to provide these services.
Claro -Revolutionary Rechargeable Hearing Aids
- Automatic shutdown when charging
- LED indicator of charging status
- 3 listening programs,Telecoil available
- 4 Channel digital chip WDRC Multi Memories Amplifier
Ask Us Anything
Definitely. There are numbers of hearing aid suppliers in China, we may not be the best, but we’re certainly trustworthy.
AZ Hearing is a professional manufacturers and factory of hearing aids, sound amplifiers and faceplate in China, we have over 6 years of OEM/ODM experience.
As a medical device, hearing aid is closely related to the user’s health and life, and quality is especially important. Quality is a key part enriches us with confidence. We share information and do business with our partners openly and honestly.
Hearing aids are relatively small in size, light in weight and high in added value, and express delivery is the preferred method for most buyers, with express carriers including DHL, UPS, FedEx, TNT and others. The shipping fee for the sample is around $35$-60$ based on global zones for your reference.
If you have set up your own express account, we could ship with your account if you wish.
Good question, thank you for asking it. Hearing aid manufacturing in China has developed rapidly in the last decade or so. The market need for hearing aids is increasing with population ageing and awareness of hearing care. From the demand side, hearing aids market is thriving.
Hearing aid technology is also evolving, for instance, the application of Bluetooth, apps and rechargeability. We think hearing aids will become an increasingly important wearable device that can assist users during daily life.
The involvement of tech companies like Bose, Nuhera & Eargo in recent years indicates that more and more companies are bullish on hearing aid market.
We’re looking forward to more changes in hearing aids market and will continue to offer cost-effective hearing aids for our partners.
First of all, our factory follows ISO regulations and has a strict quality inspection process to ensure quality. IQC is performed after raw materials are obtained, FQC is performed when production is completed, and OQC is performed after packaging.
We also implement the CAPA (Corrective and Preventive Action) system to improve quality. If there are any problems occur during the production, the process will be suspended and we will provide feedback on the problem. Preventive actions will also be taken to stop similar problems happen in the future.
If you could repair locally, we can send components to you; or you can send defective hearing aids back to China so we could repair/refurbish them. China Customs has strict requirements and procedures for importing used electronics, but don’t worry, we are able to follow up to ensure a smooth process.
In short, hearing aids are medical devices, sound amplifiers are electronics. Whether they are sound amplifiers or hearing aids depends on how different markets define each type. In some European countries, if the gain exceeds 25dB could be a boundary. In other countries, hearing aids mean that programmable while sound amplifiers can be digital but not programmable.
The Resource to Help You Become Expert of Hearing Aids

Bose Hearing Aid-FDA approved OTC Medical Device
According to the latest news, Bose hearing aids were born. Let’s take a look at what they look like. The Bose Hear app is the first self-tuning mobile app that is clinically proven to provide audiologist-level customization for individuals 18 years of age or older with mild to moderate hearing loss. Bose hearing aids are currently available for purchase in MA, MT, NC, SC and TX states for $849.95.
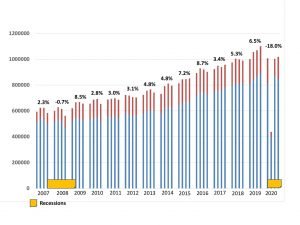
2020 Hearing Aids Sales in USA
The year 2020 has said goodbye to us, maybe you’ve gone through a long period of time where your clinics were closed, or maybe your online sales have grown in the past months instead. Now let’s take a look at 2020 hearing aid sales in the U.S. market.
What adjustment shall we make in hearing care industry with Covid 19
Hearing aid users are susceptible the most to the virus. Still, we could make some adjustments to better serve patients under this particular circumstance.
Get a Quick Quote
Any message from you will receive our prompt reply in 24 hrs
- [email protected]
- +86-15960200443
- No.466, Xinglinwan Road, Jimei District, Xiamen 361021 China
